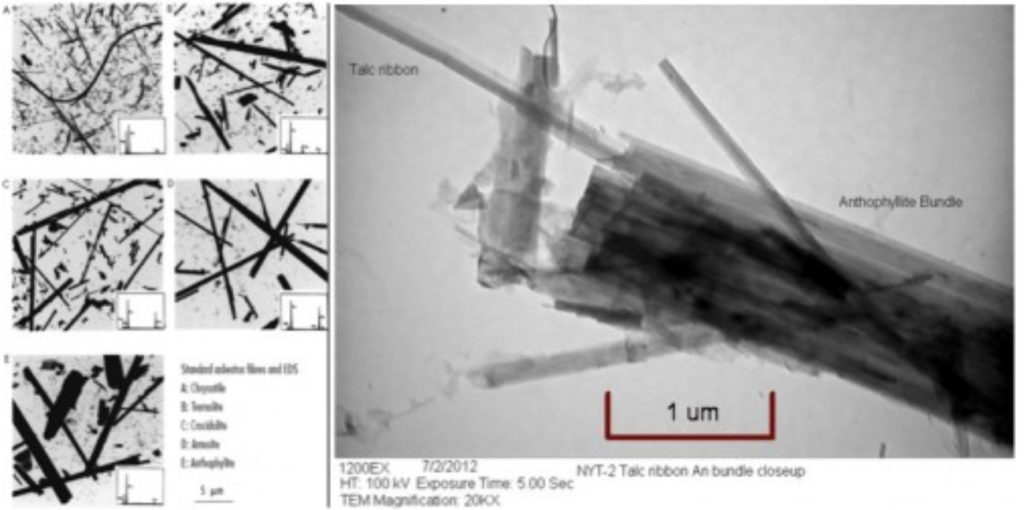
On January 12, 2022, the United States Food and Drug Administration’s Interagency Working Group on Asbestos in Consumer Products (IWGACP) released a white paper that includes recommendations on analysis methods and criteria for counting asbestos particles when analyzing for their presence within talc. This paper was produced following several years of discussion and a public hearing.
The “purity” of talc within consumer products (including cosmetics and powders) has been subject to increased scrutiny due to cases of cancer linked to use of talc-containing products that were reportedly contaminated with asbestos. The IWGACP was assembled to research existing analytical methods used by the talc industry and provide recommendations and guidance.
Some highlights of IWGACP’s recommendations include the following:
- Use both PLM and TEM methods to identify/report, at minimum, the presence of asbestos, other amphibole minerals, and talc particles exhibiting non-platy morphology.
- Tabulate, at minimum, all amphibole and chrysotile particles having a length >= 0.5 um and an aspect ratio >= 3:1 in talc-containing cosmetic products and talc intended for use in cosmetics, and avoid categorizing such particles as non-asbestiform when there is no ambiguity as to habit of growth.
- TEM results should be reported by tabulating each particle to facilitate an estimate of the number of particles per unit mass of sample analyzed (particles/gram), rather than weight percent.
- Policies and procedures to ensure laboratories are qualified and qualifications are reviewed regularly.
Links to published document and Appendices:
White Paper text
Appendices